The Evolution of Pathology: Embracing the Digital Frontier
For over a century, traditional pathology has stood as the bedrock of disease diagnosis, fundamentally relying on the meticulous microscopic examination of glass slides. While undeniably effective and deeply entrenched in medical practice, this method inherently presents limitations concerning accessibility, reproducibility, and the efficient sharing of critical diagnostic information across diverse geographical locations.¹ These constraints have, over time, underscored the need for a more dynamic and interconnected approach to diagnostic medicine.
The convergence of digital pathology signifies a fundamental re-imagining of how pathology is conducted, driven by the need to address the inherent limitations of traditional methods and meet the escalating demands of modern healthcare, particularly in an increasingly interconnected world where rapid expert collaboration, large-scale data analysis, and seamless information exchange are paramount for optimal patient care.
The inherent capabilities of digital images equip pathologists with more powerful and versatile tools to support their diagnostic decisions. One of the most compelling impacts of digital pathology is its profound potential to elevate diagnostic accuracy across various clinical scenarios. Digitalizing pathology workflows and data enables large-scale data storage, retrieval, and analysis, paving the way for developing robust diagnostic algorithms.
In addition, the integration of digital pathology with other “omics” technologies, such as genomics and proteomics, promises new insights into the molecular underpinnings of diseases, thereby charting a clear path toward personalized medicine.¹ Without this foundational digitalization, the full potential of these advanced diagnostic capabilities, including precision medicine and highly automated analyses, would remain severely limited or entirely unattainable at scale, underscoring its strategic importance for the long-term trajectory of healthcare diagnostics.
Unlocking Precision: How Digital Pathology Elevates Diagnostic Accuracy
Enhanced Visualization and Image Quality
Good digital pathology systems generate exceptionally high-resolution images that can be displayed on premium-quality monitors. This capability provides pathologists with an unparalleled, highly magnified, and detailed view of histological features, significantly aiding in accurately identifying subtle or very focal abnormalities that might otherwise be challenging to discern.⁴ In many instances, the intrinsic image quality produced by whole slide imaging (WSI) systems surpasses the visual fidelity achievable through a traditional microscope.¹
The key word there, however, is good digital pathology systems. The quality of the whole slide image, the sophistication of the viewing software, and the display screen’s resolution significantly affect the final image’s quality. This underscores the necessity of choosing good vendors and equipment to ensure a consistently reliable result.
The Transformative Power of AI and Machine Learning in Diagnosis
Artificial intelligence (AI) and machine learning algorithms are rapidly advancing in availability and accuracy, serving as powerful assistants to pathologists. These tools are adept at assisting in the identification, quantification, and precise characterization of a wide array of diagnostic entities. The deployment of these AI-driven tools holds significant promise for improving both intra-observer and inter-observer diagnostic consistency.⁴
AI particularly excels in detecting subtle “gaps”—minute patterns or inconsistencies within tissue samples that can be easily overlooked by the human eye, especially under time constraints. These subtle cues can be early indicators of disease or highlight areas demanding further investigation, potentially enabling earlier intervention.⁵
AI algorithms can identify minute alterations in cell morphology, intricate patterns of cell distribution, and other histopathological features that are inherently difficult to notice within practical diagnostic timeframes consistently.² While human pathologists often rely on heuristics and experience, AI can perform complex comparisons that are not readily achievable with traditional microscopy. This includes side-by-side comparisons of control and treated/diseased slides, or the co-registration and simultaneous analysis of multiple stains applied to serial sections.² Lumea’s digital pathology platform is designed for seamless integration with numerous AI companies, making these advanced tools accessible with a simple click.⁴
The tangible impact of AI is already being demonstrated; in initial testing for intestinal metaplasia, AI successfully detected approximately 5% of cases that had been missed by pathologists.⁵ Furthermore, AI-assisted reporting of specific features or lesions allows pathologists to offload repetitive tasks and concentrate their expertise on more complex and challenging cases, thereby better managing an ever-increasing workload.² A significant advantage of AI is its capacity to ingest and analyze immense volumes of data, including information aggregated from multiple institutions. This enables AI to discern broad patterns and correlations across vast datasets that would be impossible for human analysis alone.⁵
The consistent framing of AI’s role as assisting and augmenting the pathologist’s expertise, rather than replacing it, clarifies its collaborative nature. AI’s true value lies in its unparalleled ability to process vast datasets, identify subtle patterns that might escape human detection, and automate repetitive tasks. This liberates pathologists to dedicate their unique skills and nuanced judgment to the most complex cases, ultimately leading to a more robust and accurate diagnostic outcome through this synergistic partnership.
This emphasis on AI’s ability to quantify and precisely characterize features, alongside biomarker quantification, signifies a crucial shift. Digital pathology, particularly when augmented by AI, enables a more objective, data-driven, and quantitative approach to diagnosis. This transition from largely subjective visual assessment to measurable parameters significantly reduces inter-observer variability, leading to more consistent, reproducible, and ultimately more precise diagnoses. This quantitative precision is a foundational requirement for advancing personalized medicine and developing highly targeted therapeutic strategies.
Facilitating Expert Collaboration and Second Opinions
Digital pathology inherently streamlines and simplifies remote consultations and the acquisition of second opinions. This capability allows pathologists to collaborate effortlessly with colleagues and specialized experts, irrespective of their geographical locations.¹ By significantly lowering the logistical barriers to accessing diverse expertise, digital pathology directly contributes to more accurate and comprehensive diagnoses.⁴ Obtaining an off-site slide for review could consume hours or days, whereas within a digital pathology software, it could take seconds.
A recent FDA policy further supports the shift towards remote work for pathologists, stipulating that they may access a variety of scanners and monitors provided these devices are properly validated in each remote work location. Lumea’s foresight in designing its software for easy usability on handheld devices offers even greater flexibility than many other digital pathology solutions.
The convergence of features such as easier remote consultations, streamlined access to archived material, and AI’s capacity to learn from vast amounts of data, including information from multiple institutions, paints a picture of a diagnostic system that transcends individual expertise. It suggests that diagnostic decisions are increasingly informed by a broader, interconnected pool of knowledge and collective intelligence.
Digital pathology actively fosters a “network effect” within the diagnostic community. Individual pathologists are no longer operating in isolation but have become integral components of a larger, seamlessly interconnected diagnostic ecosystem. This collective intelligence, powerfully facilitated by digital tools, inherently elevates diagnostic accuracy by mitigating individual cognitive biases, reducing diagnostic blind spots, and leveraging a diverse range of expertise and extensive historical data.
Streamlined Access to Archived Data for Comparative Analysis
The digital format of archived slides dramatically simplifies their retrieval for comparison with prior pathological material. This ready access to historical data is invaluable for improving the accuracy and quality of a current diagnosis, allowing pathologists to track disease progression or confirm subtle findings. While traditional glass slides can also be archived, the practical challenges of timely physical retrieval often serve as a significant barrier to their regular use.⁴
Innovative Viewing Capabilities
Digital images offer unprecedented versatility in viewing. For example, Lumea’s technology enables the simultaneous display of multiple section levels and various special stains on one screen. Pathologists can even navigate and zoom multiple images simultaneously.⁴ This accelerates the review of all available levels and facilitates the easy tracking of a single focus of interest across different levels and various stains.
Navigating the Nuances: A Balanced Look at Diagnostic Accuracy
It is important to acknowledge that the comparative accuracy of digital pathology versus traditional pathology has not yet been exhaustively studied across all contexts, and published results have shown variability. While some studies indicate comparable accuracy between the two methods, others highlight potential advantages for digital pathology in specific diagnostic scenarios.⁴
A significant meta-analysis, encompassing 24 studies, concluded that digital pathology demonstrated “equivalent performance” compared to light microscopy for routine diagnostic purposes. This review reported an impressive overall concordance pooled percentage agreement of 98.3% (with a 95% confidence interval of 97.4% to 98.9%).⁶ Another systematic review, which included 38 studies, found a weighted mean diagnostic concordance between whole slide imaging (WSI) and light microscopy (LM) of 92.4%. Furthermore, the weighted mean kappa coefficient was calculated at 0.75, which signifies a “substantial agreement” between the two diagnostic modalities.⁷
This consistent finding of “comparable accuracy” and “equivalent performance” in meta-analyses for routine diagnostic interpretation suggests that, for fundamental diagnostic tasks, digital pathology is largely on par with traditional methods. Therefore, the improvement in accuracy largely stems from the augmented capabilities that digital pathology enables, such as AI integration, enhanced collaboration, and innovative viewing, rather than a blanket superiority in basic image interpretation.
This approach maintains credibility by framing digital pathology’s accuracy benefits not as a direct replacement or inherent superiority over the fundamental diagnostic capability of traditional microscopy in all cases, but rather as a powerful enhancement to the overall diagnostic process and outcomes. The improvement is holistic, encompassing reduced errors, improved consistency, and access to advanced analytical tools, rather than solely focusing on the raw ability to identify a single feature on a slide compared to a microscope.
Considerations Favoring Traditional Microscopy
Z-Plane Scanning: Traditional pathology offers the unique advantage of direct examination of tissue-bearing slides under a microscope, which provides the ability to focus through the third dimension of a tissue section—its thickness, or “z-plane.” Although these sections are remarkably thin, the capacity to view all levels of a tissue specimen can offer a superior evaluation of nuclear and cytoplasmic detail compared to a single-plane image that characterizes most digital scans.⁴
It is noteworthy, however, that viewing the z-plane is not a requisite for accurate diagnosis in most specimens. Experienced digital pathologists often report that they do not “miss” this capability in their routine work. For cases where it might be critical, two alternatives exist: employing a scanner that supports z-plane scanning (though this results in considerably larger file sizes and longer scanning time) or, when absolutely necessary, reverting to the examination of the original glass slide. Some studies suggest that Z-stacking functionality, particularly at higher magnifications, can indeed increase diagnostic accuracy and reduce diagnosis time for certain cytological specimens. However, this comes at a cost, as stated earlier.⁸
Familiarity: Pathologists, especially those with decades of experience in the field, possess extensive comfort and proficiency with traditional microscopy. This deep-seated familiarity and established workflow may, particularly during the initial transition phase, contribute to a higher degree of diagnostic confidence and, consequently, potentially more accurate diagnoses.⁴ This initial difference in comfort and speed is typically minimized with increasing experience and proficiency in using digital viewing software and image navigation devices. Dr. Todd Randolph, a seasoned pathologist, reported feeling comfortable reading digital cases within just 5 minutes of making the switch.
Addressing Digital Artifacts
Digital pathology, by its very nature, introduces the possibility of occasional artifacts. These can arise from various stages of the digitalization process, including image capture, display rendering, or data compression. Traditional pathology, as a direct optical method, inherently avoids these potential issues.⁴
Further challenges in digital image quality can include difficulties in maintaining consistent focus across uneven samples, accurately detecting the boundaries of specimens, achieving optimal color fidelity with appropriate brightness and contrast, and ensuring sufficient resolution and sharpness for evaluating the fine cellular details crucial for clinical pathology specimens. These issues could theoretically lead to a pathologist unintentionally overlooking subtle yet clinically significant features, such as infectious organisms or subtle toxic changes.⁸ There are great products that can improve focal plane definition, like the FocalRing® and BxAlign®, but they mean additional costs.
The Importance of High-Quality Systems and Pathologist Adaptation
The selection of high-quality whole slide image scanners, robust viewing software, and high-resolution display screens is essential. These components directly influence the visual fidelity and diagnostic comfort of the digital image, making it critical to invest in products that deliver consistently high-quality results.⁴
The transition to digital pathology is not merely a technological upgrade but a significant organizational change. It involves a learning curve for all personnel and necessitates a proactive approach to change management. Cultivating a lab culture that embraces innovation, flexibility, and a willingness to adapt is crucial for successful adoption.
Pathologists must also consider the personalization of their digital workspace, including selecting the optimal monitor, mouse, or even deciding whether to utilize a tablet instead of a traditional computer. The flexibility in building out a digital pathology workspace means individual testing is required to determine what works best for each user.
The discussion of pathologist familiarity, the learning curve, and the importance of a flexible culture underscores that successful digital pathology implementation and maximizing its accuracy are not solely dependent on the technology itself. The comfort, training, and willingness of the pathologists and lab staff to embrace new workflows directly influence diagnostic confidence and, particularly in the initial phases, can impact diagnostic accuracy.
This highlights that successful digital pathology adoption is fundamentally a change management initiative. Technology vendors must offer comprehensive support, training, and consulting services. The investment should extend beyond hardware and software to include significant resources for human adaptation and cultural transformation, ensuring the human element is prepared to leverage technological advancements fully.
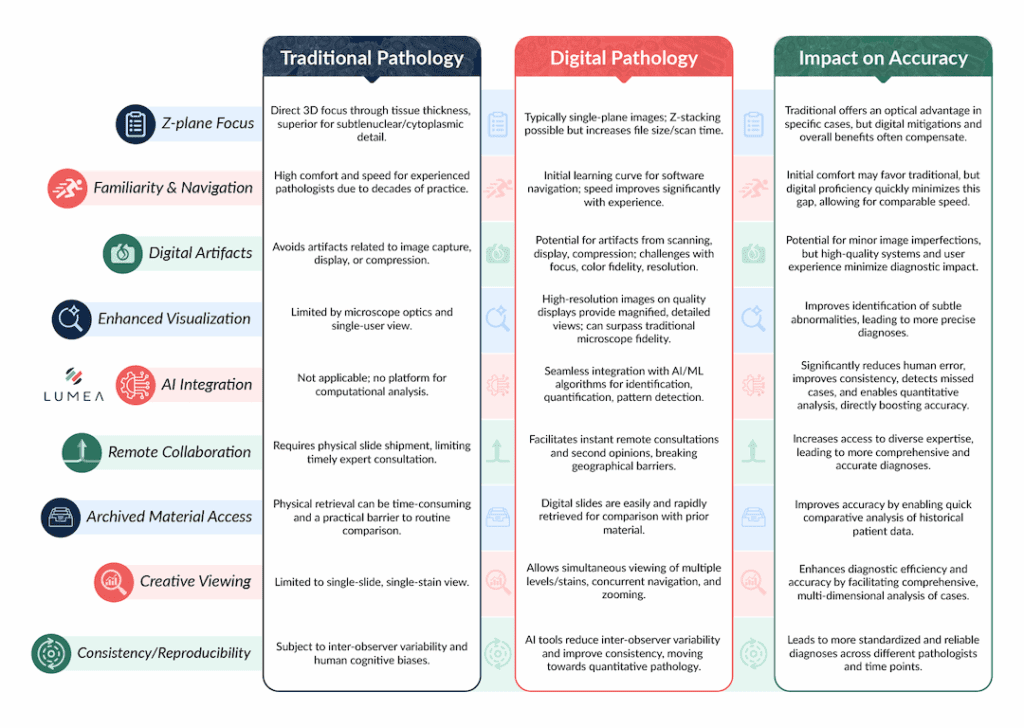
Table 1: Digital Pathology vs. Traditional Microscopy: A Comparative Analysis of Diagnostic Accuracy Factors
Beyond Accuracy: Operational Excellence with Digital Pathology
The benefits of digital pathology extend far beyond direct diagnostic accuracy, profoundly impacting operational efficiency, accessibility, flexibility, and financial viability for pathology practices.
Unprecedented Efficiency
Dr. Todd Randolph, a highly experienced pathologist with over 26 years in the field and co-founder of StarPath, made the pivotal decision five years ago to transition to Lumea’s digital pathology LIS, BxLink™, a one-stop shop for everything urology. His experience revealed profound improvements in efficiency, accessibility, and flexibility compared to his previous traditional pathology workflow.
From the outset, Dr. Randolph was impressed by how Lumea’s LIS software matched and significantly enhanced his existing workflow. Instead of a desk cluttered with stacks of cases, slides, and paperwork, BxLink allowed him to easily organize and search cases, proving “more efficient than if you’re in a traditional pathology setting.”
Within BxLink’s slide viewer, pathologists can view patient information and slide labels simultaneously, ensuring seamless verification and reducing the need to toggle between different systems. Unlike many other digital pathology software solutions requiring multiple monitors for diagnosing histologic preparations, Lumea’s intuitive design means pathologists only need a single screen, offering unparalleled versatility.
Dr. Randolph noted, “Lumea can personalize BxLink to you throughout the entire process. This leads to very efficient, real-time sign out.” With the profound efficiencies powered by Lumea, he halved his diagnostic time.
Enhanced Accessibility: Breaking Geographical Barriers and On-Demand Access
Once the staining and imaging processes are complete, BxLink instantly makes cases accessible, liberating pathologists from the traditional delays of waiting for couriers to deliver glass slides. This immediate availability allows pathologists to commence their daily caseload at any time, unconstrained by physical delivery schedules.
Furthermore, the platform enables access to cases from multiple locations. Dr. Randolph highlights this, stating, “If a clinician would like me to review a particular case, I can easily search for that within this application and have the slide ready to review in a matter of seconds.” He contrasts this with his prior experience: “Before BxLink, if a case had been sent back to histology or the laboratory, it might take an hour or a day for me to get it before I could review it again. That’s been a huge advantage.”
Unmatched Flexibility: The Lifestyle Advantages for Pathologists
“If we’re focusing on the lifestyle advantages of BxLink, it’s that it’s portable,” remarked Dr. Randolph. As long as they are in a validated location with a validated device, a pathologist can work virtually anywhere, anytime.
Dr. Tiffani Milless of Goldfinch Laboratory profoundly emphasizes the personal, unexpected benefit of increased flexibility, which has revolutionized her ability to achieve a better work-life balance as a mother to young children. She can now sign out cases from home after her children are asleep, eliminating the need to drive back to the lab. This allows her to be present for the important parts of her life while still providing critical medical care.
Beyond individual lifestyle benefits, this flexibility also opens up unprecedented opportunities to serve underserved areas of the world that need more pathologists. Dr. Randolph further underscores the profound advantages of Lumea’s platform, noting that it eliminates the need for complex interfacing between a digital viewing system and a non-digital reporting system, leading to “tremendous efficiencies and accuracy with patient tracking from bedside/specimen procurement to image production and report generation.”
Scaling for Success
Goldfinch Laboratory challenged the traditional notion that general practitioners are necessary to fulfill access-to-care obligations in rural areas. Instead, they believed they could deliver specialized care remotely with the right technology and a unified team. The first significant challenge was selecting a scanner and viewer.
After experiencing “demo exhaustion” from evaluating numerous options, they sought reliable advice from digital pathology experts and found Lumea. Lumea’s software was a perfect fit, offering existing integration with LigoLab, scanner agnosticism, and the necessary customization and flexibility. Digital pathology optimized their entire workflow, creating a virtuous cycle where improvements in efficiency, accessibility, and flexibility ultimately lead to superior diagnostic outcomes and enhanced patient care. This comprehensive value proposition is a powerful differentiator for their lab.
The Goldfinch Laboratory’s ability to scale rapidly on a “shoestring budget” through volume-based payment models showcases a new, financially viable paradigm for pathology labs. This positions digital pathology as more than just a lab upgrade; it’s a strategic tool for addressing systemic healthcare challenges. It democratizes access to specialized pathology services, fosters innovative business models (like remote subspecialty practices), and provides a scalable solution for critical issues such as pathologist shortages and geographical disparities in healthcare access. This broader societal impact enhances the value proposition of digital transformation.
For pathology labs contemplating digital transformation, the choice of a technology vendor extends beyond product features. It encompasses the vendor’s role as a trusted partner, offering comprehensive consulting, implementation support, and a deep understanding of digital pathology’s technical and operational nuances. Lumea’s demonstrated capability as an integrator and advisor is a significant competitive advantage, reducing risk and accelerating successful adoption for its clients.
Goldfinch and Dr. Randolph’s stories reveal a profound, indirect causal link back to diagnostic quality. The halved diagnosis time, instant accessibility of cases, and newfound flexibility for pathologists (reducing fatigue, enabling remote work-life balance) all contribute to a more optimal diagnostic environment.
Lumea’s Role in Driving Pathology Innovation
Lumea offers a comprehensive and interconnected suite of solutions meticulously designed to address every critical aspect of digital pathology. Lumea’s core strength lies not merely in the individual excellence of its products but in its ability to provide or facilitate a cohesive, integrated digital pathology ecosystem. This holistic approach significantly reduces operational friction, minimizes potential errors arising from data transfer or manual reconciliation, and alleviates administrative burdens. This directly contributes to enhanced efficiency, and by extension, improved diagnostic accuracy and overall lab performance. It also offers a “single pane of glass” experience for pathologists, allowing them to focus on diagnosis rather than managing disparate technologies.
Embracing the Future: Advice for Digital Adoption
While offering profound benefits, the transition to digital pathology also involves a period of adaptation and change.
While Dr. Todd Randolph reported a remarkably quick personal adaptation, feeling comfortable reading digital cases in just 5 minutes, Dr. Tiffani Milless provides a more generalized and crucial perspective, advising that “there’s definitely a learning curve associated with digital pathology adoption—it’s different than using a microscope.”
Successful digital transformation necessitates proactive and empathetic strategies. This includes providing comprehensive training, fostering an environment that values continuous learning, and cultivating adaptability within the workforce. It reinforces the value of a supportive partner, suggesting that support extends beyond product delivery to include crucial human-centric guidance for a smooth transition.
Dr. Milless strongly emphasizes that implementing digital pathology is a “major change management initiative” that must be rooted in the lab’s organizational culture. She advises patience and a strategy of starting small, encouraging everyone to speak up and share their experiences to foster collective learning. Digital adoption, she warns, “isn’t going to be easy for people that are very rigid, old school, and unwilling to change. It requires flexibility, curiosity, and a willingness to grow and look at things differently.”
While there is an undeniable upfront investment in time, effort, and temporary discomfort associated with training and adaptation, the long-term benefits—such as enhanced efficiency, improved quality, and reduced workload—ultimately create a user experience so superior that the initial resistance transforms into staunch advocacy. This provides a powerful and reassuring message for prospective adopters: the initial learning curve and associated challenges are temporary and represent a worthwhile investment. The enduring benefits of digital pathology, both operationally and in terms of staff satisfaction and retention, far outweigh the transient discomfort of the transition. It positions digital adoption as an investment in a significantly better and more sustainable future state for the pathology practice.
The Future of Diagnostics is Digital: And it’s Already Here
Digital pathology is fundamentally transforming diagnostic medicine, moving beyond traditional limitations to enhance workflow efficiency, enable large-scale data storage and sophisticated analysis, and pave the way for developing highly robust diagnostic algorithms.¹ Beyond its direct impact on accuracy, digital pathology offers unprecedented gains in operational efficiency, expands diagnostic accessibility, and provides unparalleled flexibility for pathologists.
Crucially, digital pathology addresses several critical challenges facing modern healthcare systems, including the shortage of pathologists, the urgent need for subspecialty expertise in rural and underserved areas, and the imperative for cost optimization within laboratory operations.
The cumulative weight of evidence presented collectively positions this technology as an indispensable tool for modern healthcare. Pathology labs and broader healthcare systems that hesitate or fail to embrace this transformative transition risk falling behind in terms of diagnostic quality, operational efficiency, ability to attract and retain top talent, and capacity to meet evolving patient needs.
Discover Lumea: Request a Demo Today!
Interested in learning more about how digital pathology can positively impact your practice? Request information today about our unique product lines, like our histology tech that improves specimen quality downstream for better diagnostic accuracy, our all-in-one LIS, or our consulting services. You can also try our IMS/Viewer for free today.
Works cited
-
Transforming Diagnostics: A Comprehensive Review of Advances in Digital Pathology, accessed July 1, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11573928/
-
Advancements in pathology: Digital transformation, precision medicine, and beyond – PMC, accessed July 1, 2025, https://pmc.ncbi.nlm.nih.gov/articles/PMC11910332/
-
Transforming Diagnostics: A Comprehensive Review of Advances in Digital Pathology, accessed July 1, 2025, https://pubmed.ncbi.nlm.nih.gov/39564069/
-
Is Digital Pathology Less Accurate Than Traditional Pathology? – Lumea, accessed July 1, 2025, https://lumeadigital.com/digital-pathology-accuracy/
-
Leveraging AI to Transform Pathology, accessed July 1, 2025, https://pathology.duke.edu/blog/leveraging-ai-transform-pathology
-
Diagnostic concordance and discordance in digital pathology: a systematic review and meta-analysis – Bohrium, accessed July 1, 2025, https://www.bohrium.com/paper-details/diagnostic-concordance-and-discordance-in-digital-pathology-a-systematic-review-and-meta-analysis/812569309959159810-5128
-
The Diagnostic Concordance of Whole Slide Imaging and Light Microscopy – Semantic Scholar, accessed July 1, 2025, https://pdfs.semanticscholar.org/3bfb/0679fe45211869c476a292fafa7d1ae579b3.pdf
-
Applications and challenges of digital pathology and whole slide imaging – ResearchGate, accessed July 1, 2025, https://www.researchgate.net/publication/276356817_Applications_and_challenges_of_digital_pathology_and_whole_slide_imaging

