Driving innovation across every step of the pathology workflow to increase efficiency and improve every life it touches
Built for Better
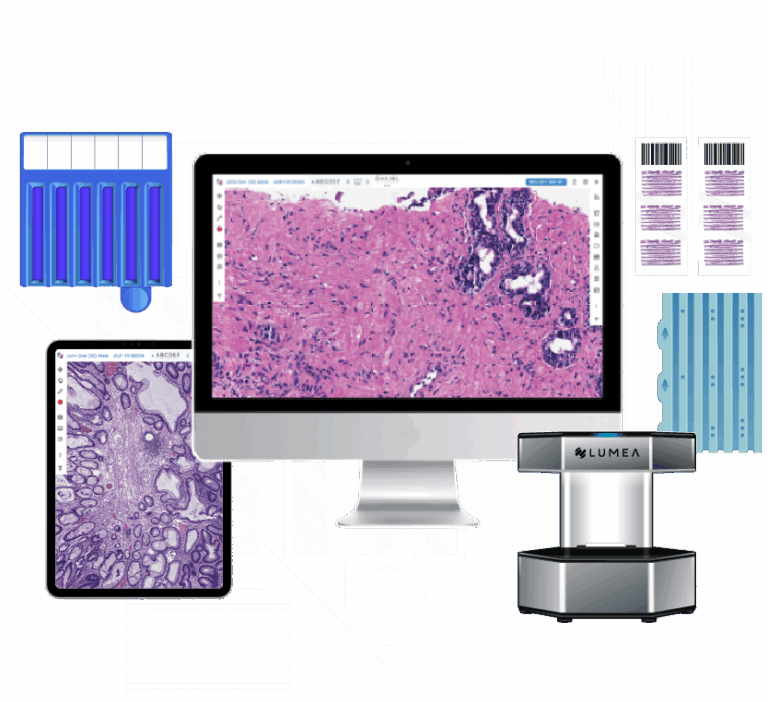
A digital pathology workflow that begins with the patient at biopsy, delivering better labs, better diagnostics, and better data.
Better Lab Technology
Make lab techs’ lives easier while boosting efficiency and revenue.
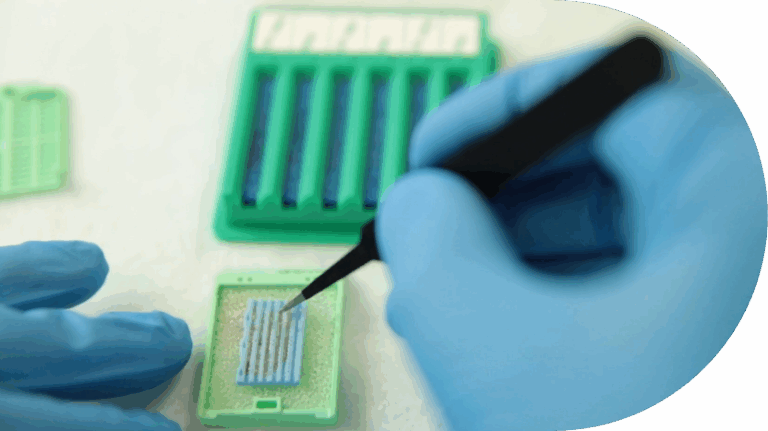
Lumea’s tissue technology improves sample quality, speeds turnaround, enhances RNA testing, and increases cancer detection while reducing time, cost, and complexity.

Better Digital Pathology
A smarter, faster, FDA-cleared diagnostic platform that integrates every diagnostic tool, including one-click molecular testing and access to an open AI marketplace, all on a single screen.
Real Results. Real Impact.
Trusted by top urology, GI, and derm groups nationwide to deliver what generic systems can’t: performance that scales.






“Lumea is perfectly positioned to allow pathologists to enter into the digital pathology space. I hear pathologists say “we know we have to go digital but we just have no idea where to even start”. Lumea bridges that gap. By providing a scaleable solution for individual pathologists and/or practices.”
Adam Cole, MDTruCore Pathology Founder & CSO
Frequently Asked Questions
I want to use digital pathology but I have no idea where to start! Where do I begin?
Get our free Digital Pathologists’ Playbook, your step-by-step guide to going digital. The booklet includes step-by-step instructions, vendor lists, specs to keep in mind, how to get a return on investment, and must-attend annual events that will keep you up-to-date on the latest news and tech.
Download it for free here: https://lumeadigital.com/digital-pathologists-playbook/
Is there ROI in digital pathology?
Yes, there is a return on investment and it can be seen in multiple facets. If you utilize certain histology and tissue technology, that investment return is typically seen within 3 months of the initial investment. If not, cost savings will be seen in the following: storage, shipping, consultations, AI analysis and efficiencies, scalability both in the lab and with pathologists, advanced analysis, physical transportation of both personnel and slides, turnaround times, streamlined and centralized and most important, improved diagnostic accuracy.
Read more about ROI in digital pathology.
Is digital pathology as accurate as traditional pathology?
Yes! There have been many research studies on the accuracy of digital pathology compared to traditional with the result of diagnosis being consistent independent of the method. It’s important to note that the accuracy of both digital and traditional pathology largely depends on the skill, experience, and training of the pathologist. Additionally, the quality and calibration of digital equipment and the digitization process can influence the accuracy of digital pathology.
Read more about digital pathology accuracy
What exactly does Lumea do?
Is Lumea limited to the urology/prostate market
No! Lumea’s IMS handles all small surgical biopsies. The IMS also integrates with our tissue technology to create never-before-scene pathology efficiencies, which we pioneered with prostate tissue but are continually expanding into other specialties.
Will digital pathology slow me down?
Like anything, change comes with a learning curve. Typical digital pathology has seen some minimal improvements, such as faster access to slides, easier consults, and additional digital tools. Lumea is invested in unleashing the full potential of digital pathology via integrated tissue technology and software to optimize pathology in ways never seen before.
Read more about digital pathology speed
Read more about digital pathology efficiency
What measures are in place to ensure the security and privacy of patient data, especially in compliance with US regulations?
Digital pathology can be designed with robust security measures to protect patient privacy and prevent unauthorized access in accordance with HIPAA, HITECH, and other relevant requirements. Modern encryption and authentication methods allow for secure connections, even over the internet, between web browsers and applications. Vendors should have the documentation they can provide upfront to prove compliance, but if that documentation does not satisfy your questions you should discuss it with their Information Security Officer. An important note: Your practice will be responsible for the compliance and security of the devices you use for pathology, such as securing work computers and keeping passwords safe.
Is digital pathology too expensive?
The cost of implementing digital pathology can vary widely depending on several factors, including the scale of the operation, the specific technology chosen, and the location and resources of the healthcare institution. While there are material upfront costs associated with digital pathology, consider the potential long-term benefits, such as increased efficiency, improved accuracy, eliminated transport costs, and the ability to offer and receive rapid remote consultations. A well-implemented digital pathology system may lead to cost savings and better patient care. Here are some cost-related considerations you will want to keep in mind while contemplating digital pathology and comparing different vendors.
Read more about digital pathology expenses
Built for Better. Ready When You Are.
*Using the LDT guidelines for validation and verification.


