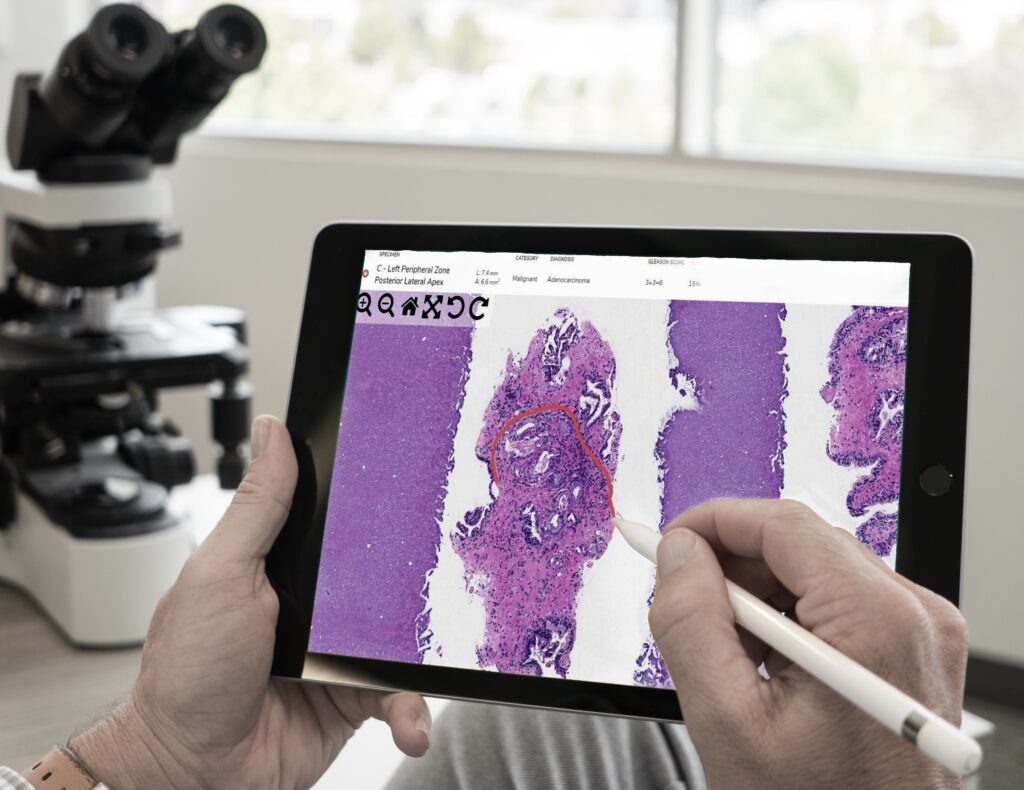
COVID-19 led to an unexpected rise in the practice of digital pathology. Temporary regulations were put in place to protect pathologists and other healthcare professionals, making diagnosing from a safe distance easier. Will these regulations remain temporary, or is digital pathology here to stay?
How has COVID-19 Affected Pathology?
The first cases of COVID-19 started in early December of 2019 in Wuhan, China. By January 20, 2020, a CDC laboratory confirmed the first cases in the United States. On March 13, President Donald Trump declared a national emergency. An unfortunate side effect of the pandemic was how it affected pathology.
Healthcare workers put their health and the health of their loved ones at risk just by going to work. The healthcare industry became short-staffed, including clinical laboratory staff and pathologists. However, there was still a great need to process and diagnose specimens so cancer patients could get the treatment they needed. The question soon became: how can we still provide quality patient care while protecting valiant healthcare workers better? As many people as possible needed the option to work remotely.
Temporary CMS Digital Pathology CLIA Waiver
The same day the US President declared a national emergency, the College of American Pathologists sent a letter to CMS. They asked for more regulatory flexibility so they could safely social distance at home.
On March 26, 2020, CMS issued a temporary digital pathology CLIA waiver. The waiver allows pathologists to view and diagnose pathology slides remotely. The main requirements are that the primary diagnostic location has a CLIA certification and the laboratory uses validated whole slide imaging systems.
Temporary Digital Pathology FDA Policy
On April 24, 2020, the Federal Drug Administration enforced a new digital pathology FDA policy. It increased the availability of whole slide imaging devices for remote diagnosis of slides. The policy made digital pathology more accessible, flexible, and less expensive. Pathologists have access to a greater variety of scanners and monitors so long as they validate the devices in each remote work location.
Is Digital Pathology Here to Stay?
The CLIA waiver and FDA policy enabled pathologists to continue to serve patients while reducing their risk of exposure to COVID-19. Both are temporary because they address issues that arose from the pandemic. The question now: is digital pathology here to stay?
Remote pathology was already in full swing in many countries before the pandemic. However, the over-regulation of whole slide imaging devices has been a barrier to adopting this technology in the United States. It took a pandemic to relax regulations enough to make digital pathology more feasible.
Will the regulations revert after the pandemic? Or will CLIA and FDA see the success of digital pathology and make some permanent changes? Share what you think in the comments below.