This FAQ answers questions about digital pathology including what is digital pathology, how does it work, and is it FDA approved.
What is digital pathology?
Digital pathology is analyzing, storing, and sharing pathology information online. Its components include a scanner, viewer, image or case management software, and a computer monitor.
How does digital pathology work?
Lab technicians scan glass slides using whole-slide imaging scanners. A pathologist can then view, manage, share, and analyze the digitized slides on a computer monitor in addition to or as a replacement for a microscope. The pathologist will use digital pathology software to view, store, manage digitized slides, and share the images with colleagues. Analysis tools help them study and diagnose the digitized slides on a computer. Their case management software can integrate with or replace existing workflows, like a Laboratory Information System (LIS), to make the process as seamless as possible. Finally, they create a report of their analysis that they send to the physician.
Is digital pathology approved by the FDA?
Yes! On April 24, 2020, the Federal Drug Administration enforced a new digital pathology FDA policy. It increased the availability of whole slide imaging devices pathologists can use to diagnose slides remotely. The policy made computational pathology more accessible, flexible, and less expensive. Pathologists have access to a greater variety of scanners and monitors, so long as they validate the devices in each remote work location.
Why is digital pathology important?
Digital pathology is important because it results in better patient care. It enables easier collaboration with colleagues, increased flexibility since they can use a computer instead of a microscope, rapid accessibility to cases and slides from a vast electronic archive, and improved efficiency and productivity. There are many other benefits of computational pathology like better ergonomics, fewer errors, reduced turnaround times, infinite scalability, and access to artificial intelligence assets.
What are some disadvantages of digital pathology?
A few disadvantages and limitations of digital pathology are the cost, lack of standardization, not all software being user-friendly, and it adds steps to a pathology and lab workflow.
Most technology focuses on what happens after the scanner. That is an essential part of our process at Lumea. However, Lumea, and other companies like us, realized early on that to make computational pathology work the entire process needs an update beginning at the biopsy. From revolutionary tissue-handling technologies to time-saving automated grossing tools, check out Lumea’s digital pathology technology for prostate cancer.
What is the future of digital pathology?
Artificial intelligence companies are increasing rapidly with tools and technologies to improve a pathologist’s workflow. Artificial intelligence can improve diagnoses with its ability to detect patterns invisible to the human eye. It can also assist with menial but essential tasks that take a significant workload off the pathologist, allowing more time to focus on interpreting data for more cases. Now patients can have faster and more accurate results, leading to better treatment plans.
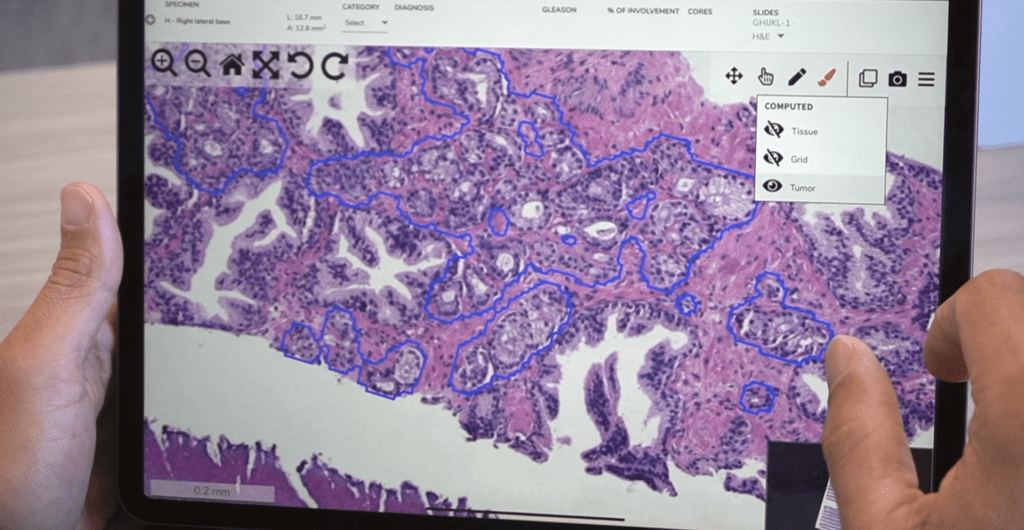
Computational pathology software began in the 1960s and has come a long way. What are you most looking forward to in the future of pathology?
Interested in learning more about what Lumea can do for your practice? Request a free demo here.

